Protein
Proteins are polymers of amino acids joined together by peptide bonds.
Each of these amino acids has a fundamental design composed of a central carbon (also called the alpha/chiral carbon) bonded to:
- a hydrogen
- a carboxyl group
- an amino group
- a unique side chain or R-group
Peptide bonds are formed between the carboxyl group of one amino acid and the amino group of the next amino acid. Peptide bond formation occurs in a condensation reaction involving loss of a molecule of water. The head-to-tail arrangment of amino acids in a protein means that there is a amino group on one end and a carboxyl group on the other end.
Levels of Protein Structure
Structural features of proteins are usually described at four levels of complexity:
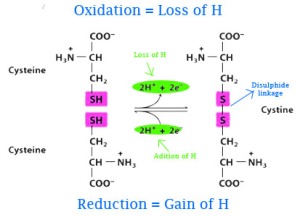
- Primary structure: the linear arrangement of amino acids in a protein and the location of covalent linkages such as disulfide bonds between amino acids. In the primary structure, it tells you what amino acid is present and the sequence of that amino acid.
- Secondary structure: areas of folding or coiling within a protein; examples include alpha helices and pleated sheets, which are stabilized by hydrogen bonding.
- Tertiary structure: the final three-dimensional structure of a protein, which results from a large number of non-covalent interactions between amino acids.
- Quaternary structure: non-covalent interactions that bind multiple polypeptides into a single, larger protein. Hemoglobin has quaternary structure due to association of two alpha globin and two beta globin polyproteins.
Summary 🙂
1. Primary
Animo acid sequence
2. Secondary
Alfa helix
Beta plates
Hydrogen bonds with the polypeptide backbone
3. Tertiary
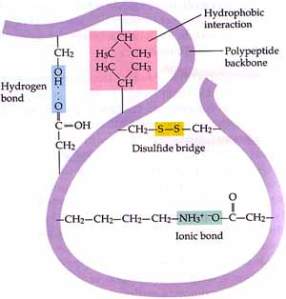
Basic structure of the protein; alpha helixes and beta plates arranged in the protein
Hydrogen bonds, Ionic bonds, Covalent bonds, Sulfide bonds
They are important interacting with the side chains
4. Quaternary
Interactions between tertiary structures/polypeptide chain
Hydrogen bonds, Ionic bonds, Van der Waals interactions, Disulfide bridges
They are important interacting with the side chains
Denaturing Proteins
Denaturation of proteins involves the disruption and possible destruction of both the secondary and tertiary structures. Since denaturation reactions are not strong enough to break the peptide bonds, the primary structure (sequence of amino acids) remains the same after a denaturation process. Denaturation disrupts the normal alpha-helix and beta sheets in a protein and uncoils it into a random shape.
Denaturation occurs because the bonding interactions responsible for the secondary structure (hydrogen bonds to amides) and tertiary structure are disrupted. In tertiary structure there are four types of bonding interactions between “side chains” including: hydrogen bonding, salt bridges, disulfide bonds, and non-polar hydrophobic interactions. which may be disrupted. Therefore, a variety of reagents and conditions can cause denaturation. The most common observation in the denaturation process is the precipitation or coagulation of the protein.
Heat, Ultraviolet radiation, Strong Acid/bases, Urea, Some organic solvents, agitation are all conditions that can lead to denaturing a protein.