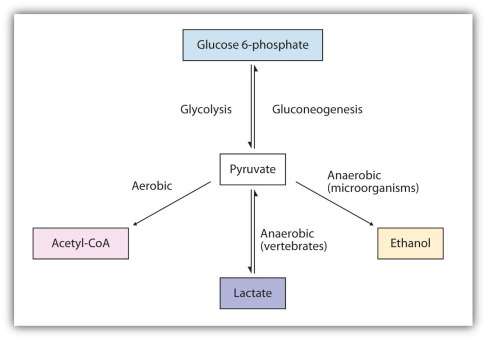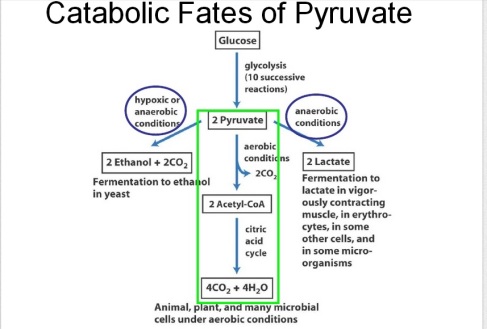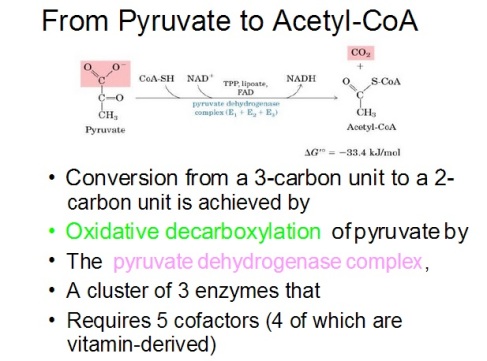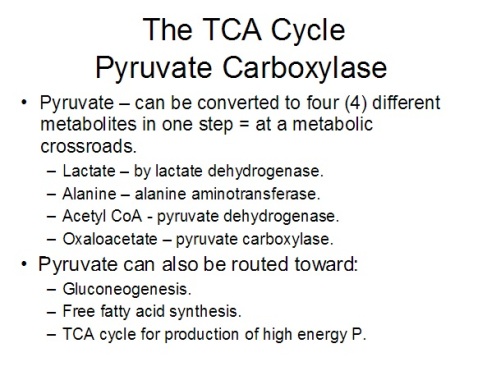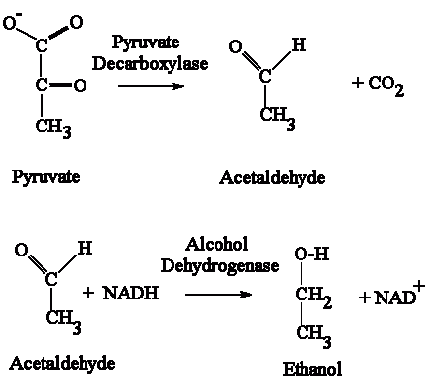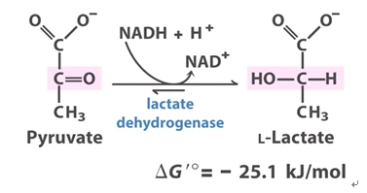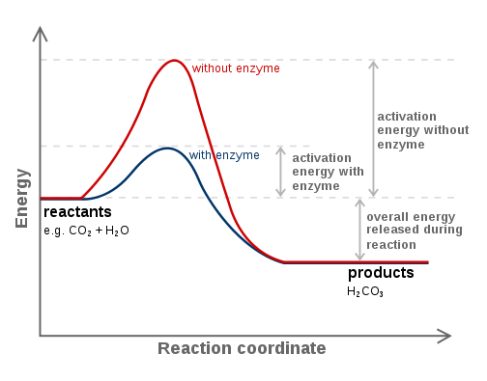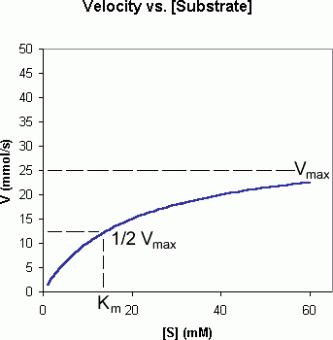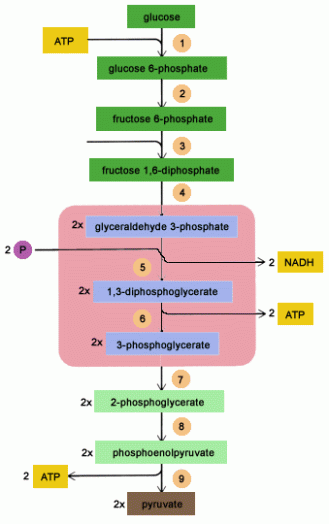
The above picture is a summary of steps in glycolysis.
Step 1
The enzyme hexokinase phosphorylates (adds a phosphate group to) glucose in the cell’s cytoplasm. In the process, a phosphate group from ATP is transferred to glucose producing glucose 6-phosphate.
Glucose (C6H12O6) + hexokinase + ATP → ADP + Glucose 6-phosphate (C6H11O6P1)
Step 2
The enzyme phosphoglucoisomerase converts glucose 6-phosphate into its isomer fructose 6-phosphate. Isomers have the same molecular formula, but the atoms of each molecule are arranged differently.
Glucose 6-phosphate (C6H11O6P1) + Phosphoglucoisomerase → Fructose 6-phosphate (C6H11O6P1)
Step 3
The enzyme phosphofructokinase uses another ATP molecule to transfer a phosphate group to fructose 6-phosphate to form fructose 1, 6-bisphosphate.
Fructose 6-phosphate (C6H11O6P1) + phosphofructokinase + ATP → ADP + Fructose 1, 6-bisphosphate (C6H10O6P2)
Step 4
The enzyme aldolase splits fructose 1, 6-bisphosphate into two sugars that are isomers of each other. These two sugars are dihydroxyacetone phosphate and glyceraldehyde phosphate.
Fructose 1, 6-bisphosphate (C6H10O6P2) + aldolase → Dihydroxyacetone phosphate (C3H5O3P1) + Glyceraldehyde phosphate (C3H5O3P1)
Step 5
The enzyme triose phosphate isomerase rapidly inter-converts the molecules dihydroxyacetone phosphate and glyceraldehyde phosphate. Glyceraldehyde phosphate is removed as soon as it is formed to be used in the next step of glycolysis.
Dihydroxyacetone phosphate (C3H5O3P1) → Glyceraldehyde phosphate (C3H5O3P1)
Net result for steps 4 and 5: Fructose 1, 6-bisphosphate (C6H10O6P2) ↔ 2 molecules of Glyceraldehyde phosphate (C3H5O3P1)
Step 6
The enzyme triose phosphate dehydrogenase serves two functions in this step. First the enzyme transfers a hydrogen (H–) from glyceraldehyde phosphate to the oxidizing agent nicotinamide adenine dinucleotide (NAD+) to form NADH. Next triose phosphate dehydrogenase adds a phosphate (P) from the cytosol to the oxidized glyceraldehyde phosphate to form 1, 3-bisphosphoglycerate. This occurs for both molecules of glyceraldehyde phosphate produced in step 5.
A. Triose phosphate dehydrogenase + 2 H– + 2 NAD+ → 2 NADH + 2 H+
B. Triose phosphate dehydrogenase + 2 P + 2 glyceraldehyde phosphate (C3H5O3P1) → 2 molecules of 1,3-bisphosphoglycerate (C3H4O4P2)
Step 7
The enzyme phosphoglycerokinase transfers a P from 1,3-bisphosphoglycerate to a molecule of ADP to form ATP. This happens for each molecule of 1,3-bisphosphoglycerate. The process yields two 3-phosphoglycerate molecules and two ATP molecules.
2 molecules of 1,3-bisphoshoglycerate (C3H4O4P2) + phosphoglycerokinase + 2 ADP → 2 molecules of 3-phosphoglycerate (C3H5O4P1) + 2 ATP
Step 8
The enzyme phosphoglyceromutase relocates the P from 3-phosphoglycerate from the third carbon to the second carbon to form 2-phosphoglycerate.
2 molecules of 3-Phosphoglycerate (C3H5O4P1) + phosphoglyceromutase → 2 molecules of 2-Phosphoglycerate (C3H5O4P1)
Step 9
The enzyme enolase removes a molecule of water from 2-phosphoglycerate to form phosphoenolpyruvic acid (PEP). This happens for each molecule of 2-phosphoglycerate.
2 molecules of 2-Phosphoglycerate (C3H5O4P1) + enolase → 2 molecules of phosphoenolpyruvic acid (PEP) (C3H3O3P1)
Step 10
The enzyme pyruvate kinase transfers a P from PEP to ADP to form pyruvic acid and ATP. This happens for each molecule of PEP. This reaction yields 2 molecules of pyruvic acid and 2 ATP molecules.
2 molecules of PEP (C3H3O3P1) + pyruvate kinase + 2 ADP → 2 molecules of pyruvic acid (C3H4O3) + 2 ATP